The boiling point of water is measured in Kelvin, Celsius, and Fahrenheit. It’s useful to know each amount. What is the boiling point of water in Kelvin, Celsius, and Fahrenheit?
Water boils at 373.2 K (Kelvin), 100ºC (Celsius), or 212ºF (Fahrenheit). When measuring temperature, the usual units are Celsius (degree Celsius) or Fahrenheit (degree Fahrenheit). However, when reporting temperatures in Kelvin, we don’t say “degree Kelvin.”
Boiling point of water:
- Kelvin: 373.2 K
- Celsius: 100ºC
- Fahrenheit: 212ºF
Read more to learn about the boiling point of water in Kelvin, Celsius, and Fahrenheit.
What Is the Boiling Point of Water in Kelvin?

Water boils at 100ºC, and this is equivalent to 212ºF or 373.2 Kelvin. When reporting temperature measurements, people usually use Celsius (degree Celsius) or Fahrenheit (degree Fahrenheit). However, when reporting using the Kelvin, we don’t say “degree Kelvin.”
One of William Thomson’s best-known contributions—also known as Lord Kelvin and 1st Baron Kelvin—is the absolute temperature scale (measured in Kelvin).
His work was inspired by the 1800s law stating that the pressure of an ideal gas (If kept at a constant volume) changes directly with its absolute temperature (or Zero Kelvin).
Just a quick information about Ideal Gas:
An ideal gas follows the rules of the Ideal Gas Law. In simple terms, this law states that when a fixed amount of ideal gas is squeezed, its temperature increases.
One real-life application of this law is engineering, wherein engineers figure out the right containers to build for holding oxygen.
What is the boiling/condensation point of water in Kelvin? The boiling point of water on the Kelvin scale is 373.2 K. We don’t insert the degree symbol (º) or the word “degree” to refer to a specific temperature on the Kelvin scale.
Every degree unit on the Celsius scale is equal to one degree unit on the Kelvin scale. Thus, we don’t use the degree symbol when reading temperatures in this system.
It’s different from Celsius or Fahrenheit because it doesn’t have negative numbers on its scale. The lowest possible number is 0 K or absolute zero—the point at which a substance or object couldn’t get any colder or has no heat energy left.
Kelvin is rarely used in daily life activities, like boiling water for your coffee and cooking. Instead, it’s extensively used in the scientific community, specifically by physicists and chemists, for some formulas.
How to Stay Safe When Boiling Water in the Microwave
What Is the Boiling Point of Water in Celsius?
The lay public popularly uses Celsius in different parts of the world. That’s not the case in the United States, where it’s mostly used by people in the engineering and scientific fields.
Celsius, which was previously called centigrade, is the Swedish astronomer and physicist Anders Celsius’s brainchild. He proposed that the temperature of boiling water depends on atmospheric pressure, among others.
The Earth is enveloped by a layer of gas, or what we call the atmosphere. This gaseous envelope has weight and would exert a downward force on everything underneath it. This pressure is called atmospheric pressure.
Atmospheric pressure affects the boiling point of water (or other liquids). If the atmospheric pressure increases, more energy is necessary to boil water. This also causes the boiling point to be higher.
What is the boiling point in degrees Celsius of water?
Originally, Anders Celsius assigned zero to stand for water’s boiling point and 100 to stand for water’s freezing point.
This was later reversed by Carolus Linnaeus (or Carl Linnaeus), a Swedish botanist, physician, and zoologist after Anders died. So, 100ºC is now the boiling point of water, while 0ºC is the freezing point of water.
Advantages of Using Celsius Rather than Fahrenheit:
- At standard temperature (refers to 0° C or 15° C at sea level), Celsius is great for measuring water temperature using its 0 to 100 scale—for instance, when you heat milk. Since milk has a lot of water, it has the same boiling point as water, approximately 100° C (depending on the atmospheric pressure);
- Celsius is part of the metric system, which has been adopted by different countries worldwide. This makes it easier to communicate daily temperature measurements to people in other countries;
- It’s simpler to convert temperature readings from Celsius to another temperature scale, like Fahrenheit; and
- Zero is the freezing point, and 100 is the boiling point. That’s easy to remember, right?
What Is the Boiling Point of Water in Fahrenheit?
Today, the Fahrenheit temperature scale is famous in a few Caribbean countries (e.g., the Cayman Islands, Bahamas, and Belize) and the United States. Daniel Gabriel Fahrenheit, a German physicist as well as engineer, developed this in 1724.
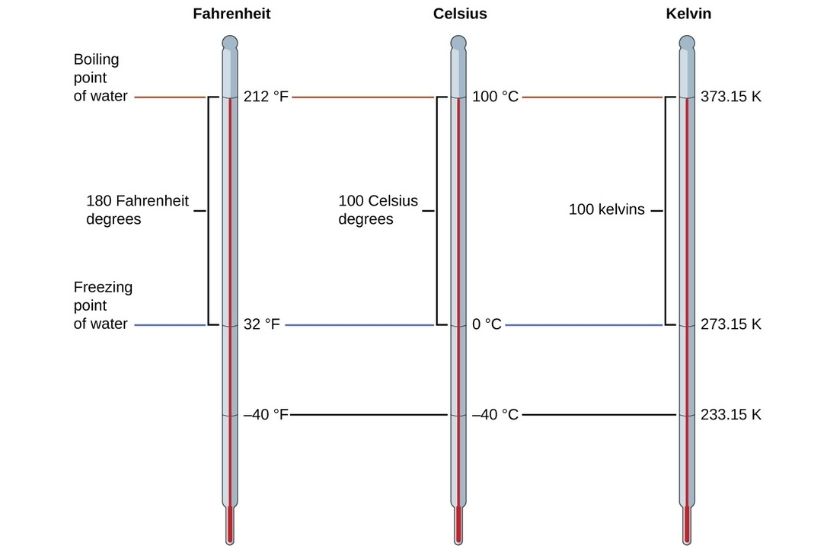
In Celsius, the boiling and freezing points of water are 100° and 0°, respectively. In Fahrenheit, those would be 212° and 32°.
He came up with this temperature scale based on the three fixed temperature points of the human body, freezing water, and a mixture of ice, salt (ammonium chloride), and water. These three temperature points were believed to be based on Rømer’s system.
During the early 18th century, Ole Roemer—the Danish astronomer who first measured the speed of light—used alcohol-in-glass thermometers to create his own temperature scale.
He assigned 60 as water’s boiling point, 22.5 as the body’s temperature, 0 degrees as the lowest point, and 7.5 as the freezing point of pure water.
So, Gabriel Fahrenheit decided to expand on Roemer’s scale system since his mercury thermometer is precise. What he did was he multiplied each value by four.
Advantages of Using Fahrenheit over Celsius
Celsius is great for measuring water temperature. However, it’s widely believed that Fahrenheit has nearly double the accuracy of Celsius. You can read the temperature on the Fahrenheit scale more accurately without using decimals or fractions since it has more degrees.
Another advantage of Fahrenheit is it’s usually better for measuring air temperature (than water). As a result, it’s an ideal rough guide for reporting the weather. Based on this temperature scale, you can relay 100 as scorching weather, 50 as mild weather, and 0 as freezing weather.
Factors That Determine the Boiling Point of Water
Three main factors affect the boiling point of water (or any liquid): atmospheric pressure, vapor pressure, and temperature. Let’s discuss these in greater detail:
1. Atmospheric Pressure
The boiling point of water at standard atmospheric pressure is 212ºF (100ºC). As defined earlier, atmospheric pressure or air pressure is the force applied against a liquid’s surface.
As a thumb rule, the higher the atmospheric pressure, the more heat energy is necessary to boil a liquid—or in this case, water. This, in turn, raises the boiling point.
If you were to take a microscopic view of this process, you’d see air molecules (the smallest particles of an element or a substance) running into the surface of the water. This creates pressure that’s spread all over the water surface.
If the pressure is high, it will be difficult for water to turn into vapor and form bubbles. Thus, water doesn’t boil. However, if this pressure is decreased, less heat energy is necessary to move water into the vapor phase. Plus, water can boil at a lower temperature.
The boiling point of water will also depend on your location. Altitude, or the height above sea level, is closely linked with atmospheric pressure. The higher you get above sea level, the thinner the air becomes. The “thin” air has lower pressure and oxygen because of the effect of gravity.
At sea level, water usually boils at 212°F (100C°). However, at higher altitudes and lower atmospheric pressures, water boils at a lower temperature.
For every increase in altitude of 500 feet, the water boiling point decreases by under 1°F. For example, at 5,000 feet (1524 meters), water boils at 202°F (94.5°C). Or, at 9500 feet (2895 meters), water boils at 194°F (90°C).
2. Vapor Pressure
Vapor pressure is the result of the evaporation of water or other liquids. It measures the ability of a liquid to transform from a gas state into a vapor state.
In case you’re wondering, vapor and gas have a few differences. For example, unlike vapor, gas isn’t solid or liquid in its natural state, and it can’t transform back into any of those states. It remains to be a gas in its natural state at room temperature.
Vapor pressure and the boiling point of a liquid (at atmospheric pressure) have an inverse relationship. So, if water’s vapor pressure at a given temperature is high, the water’s normal boiling point goes down.
The boiling point is low if the vapor pressure is high because less energy is necessary for a liquid to match the gas’ pressure above it as it’s being heated.
3. Temperature
The temperature of the water will affect the vapor pressure. It would be best if you got the water hot enough to increase the speed of its particles.
By increasing the temperature of the water, more water particles will, at some point, gain sufficient energy to change into vapor, causing bubbles to form within the water and reach the surface.
These three (atmospheric pressure, vapor pressure, and temperature) are the main factors that affect the boiling point of any liquid. Other things could increase or decrease a liquid’s boiling point, such as the addition of nonvolatile solutes.
4. Addition of Nonvolatile Substances
In general, nonvolatile solutes or substances increase the boiling point because they don’t allow vapor pressure in solutions. Two examples of these substances include salt and sugar.
Salt

Salt doesn’t really cause a drastic increase in the boiling point of water. The increase is only around 32.9ºF (0.5ºC) for every 58 grams of salt per 1 kilogram of water.
So, if you’re adding 2 grams (0.5 teaspoons) of salt to a liter of water, it wouldn’t really have a big effect on the boiling point. Now, if you’re going to add 20 grams (4.8 teaspoons) of salt, it would increase the boiling point of 5 liters of water from 212ºF (100ºC) to around 212.72ºF (100.4ºC)
Sugar
Dissolving sugar in water increases the boiling point and decreases the freezing point. But just like salt, it doesn’t cause a significant increase in the boiling point of water (approximately 32.18ºF to 32.54ºF or 0.1ºC to 0.3ºC).
Other people also believe that baking soda and pepper may raise the boiling temperature of the water. They can, but only slightly. Pepper has the least effect on the boiling point because it doesn’t really dissolve well in liquids.
Can Water Be Hotter than Its Boiling Point of 212°F (100°C or 373.2 K)?
It can. The process is called superheating. Superheated water is pressurized water, and it can reach the critical temperature of 705°F or 374°C.
However, you don’t want to heat pure and bubble-free water above its normal boiling point because it could cause it to explode out of its container when it’s disturbed.
How to Avoid This?
- If you’re using an oven to heat water, you could prevent superheating by not using a container with a very smooth surface. A container with at least some scratching would suffice;
- You can also use a long, solid object to lightly tap the outside of the container while it’s still in your oven. Please keep your hand outside the oven; and
- Of course, don’t heat it above its normal boiling temperature.
Conclusion – What Is the Boiling Point of Water in Kelvin, Celsius, and Fahrenheit?
Water boils upon reaching the 100ºC temperature. And this is equivalent to 212ºF or 373.2 K (Kelvin).
Boiling point of water:
- Kelvin: 373.2 K
- Celsius: 100ºC
- Fahrenheit: 212ºF
You’ll notice that people usually use Celsius (degree Celsius) or Fahrenheit (degree Fahrenheit). However, when reporting a temperature measurement using the Kelvin unit, we don’t say “degree Kelvin.”
Again, the boiling point of water in whatever unit (Celsius, Kelvin, and Fahrenheit) is influenced by many factors. Atmospheric pressure, vapor pressure, and temperature are three of the main ones.
In the end, the boiling point of any liquid is all about the pressure exerted on its surface, not just the temperature.
Read next:

![What Is a Comfortable Dew Point? [Comfortable Dew Point Range] comfortable dew point](https://howchimp.com/wp-content/uploads/2021/04/comfortable-dew-point-300x200.jpg)
![Read more about the article Is Stainless Steel Oven-Safe? [Pros and Cons]](https://howchimp.com/wp-content/uploads/2020/08/is-stainless-steel-oven-safe-300x200.jpg)

